Buy Human Platelet Lysate (hPL) now
Request your quote
Want to learn more about our hPL products or have specific questions about their use? Don't hesitate to contact us. Our team is ready to answer all your questions and help you find the right solution for your needs.
*One free test sample per product type per institution
Our publications
- Delabie W, De Bleser D, Vandewalle V, De Prest M, Vandekerckhove P, Compernolle V, Feys HB. The Impact of Amotosalen Photochemical Pathogen Inactivation on Human Platelet Lysate Curr Stem Cell Res Ther. 2024 in press
- De Korte D , Delabie W, Feys H, Klei T, Larsen R, Sigurjónsson Ó, Sousa AP. Towards standardized human platelet lysate production in Europe: an initiative of the European Blood Alliance Vox Sang. 2024 Jan;119(1):79-87.
- Delabie W , De Bleser D, Vandewalle V, Vandekerckhove P, Compernolle V and Feys H. Single step method for high yield human platelet lysate production Transfusion. 2023 Feb;63(2):373-383.
Certificates
hPL Gold Research (REF G150R)
P20221207_Certificate of Analysis
P20230822_Certificate of Analysis
hPL Platinum Research (REF P480R)
PL20231026_Certificate of Analysis
PL20240424_Certificate of Analysis
If you cannot find your certificate in this list, please request this by filling in the contact form above.
Frequently Asked Questions
What is hPL?
Human Platelet Lysate (hPL) is a solution obtained by lysing human platelets. It contains a rich mix of growth factors, cytokines and other bioactive molecules that stimulate cell growth, regeneration and tissue repair. This makes it therefore ideally suited as a xeno-free supplement for (stem) cell culture in cell therapy production, among others. The development of our hPL Gold allows Belgian Red Cross-Flanders to make the best use of all donations, as well as making an additional contribution to improved patient care.
Why should I use Human Platelet Lysate (hPL) instead of Fetal Bovine Serum (FBS)?
hPL is made from exclusively human material, thus no animal products are involved. This not only increases compatibility with human cell culture, but eliminates the risk of zoonotic infections as with FBS. When ultimately used for clinical purposes, there is no risk of patient immunization against animal proteins and is an additional argument to the regulator for approving your cultured cells.
Choosing hPL over FBS is also recommended for research groups. Not only is hPL often more efficient than FBS, it is, above all, an important choice for the long term of your project. When translating to a clinical setting by you or third parties, the use of FBS during research is a distinct disadvantage as it necessitates a switchover from FBS to hPL. As a result, your costly research must be re-lined with hPL. Using hPL Gold from the starting line anticipates problems later in your path.
The preparation of hPL from donor to final product lies entirely with Belgian Red Cross-Flanders, thus we have excellent control over correct storage and handling of all source, intermediate and final products. We thus also guard the rights of the donor who has given express consent. During our development, production in a closed system was crucial, so the hPL never comes into contact with ambient air, whereas FBS often ends up in open systems both during collection at the slaughterhouse and during production.
Since the source product of hPL is standardized to meet strict requirements for transfusion, the batch-to-batch variation of hPL is a lot lower than for FBS. In addition, donor material from multiple donors is pooled, further reducing batch-to-batch variation.
How is Human Platelet Lysate made?
Belgian Red Cross-Flanders has developed a new method that effectively yields growth factor-rich supplement from excess platelet concentrates. This method removes platelet residue and lowers fibrinogen concentration, eliminating the need for heparin during cell culture. The new method and resulting hPL allow for optimal use of blood donations and contribute to the development and production of promising cell therapies.
Blood donation in Flanders is done exclusively by voluntary, non-remunerated donors who give their explicit consent. Donations are always used according to what is included in the informed consent. Risk behavior of donors is questioned and donations are extensively tested according to (inter)national quality requirements for transfusion. Platelet concentrates are treated with pathogen inactivation, reducing the risk of viral and bacterial transmission.
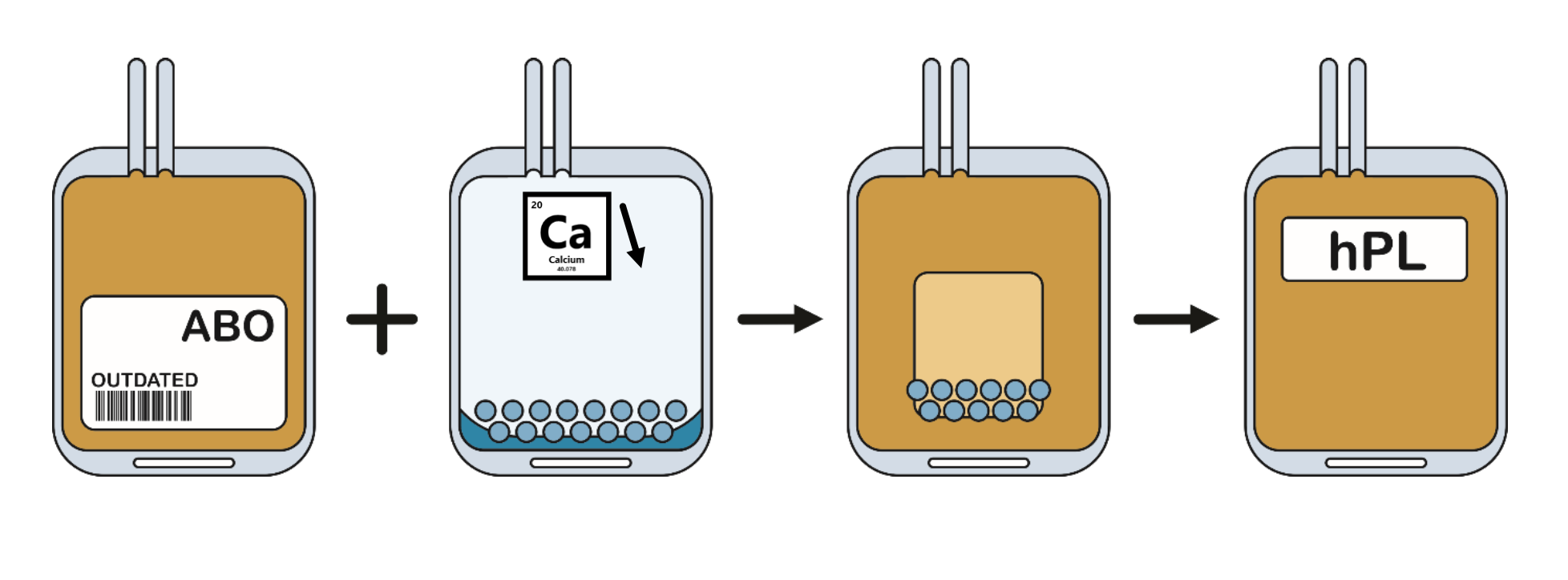
What makes the hPL of the Belgian Red Cross-Flanders so special?
All our hPL is prepared from Belgian donations (Flanders), exclusively from voluntary, non-renumerated donors who give their consent. The preparation of hPL from donor to final product lies entirely with Belgian Red Cross-Flanders, thus we have excellent control over correct preservation of all source, intermediate and final products.
Production in a closed system was central to the development, this ensures that the hPL never comes into contact with the ambient air which is often a risk in industrial preparations. The closed system, combined with high grade source material and extensive testing results in high safety.
During preparation of both hPL Gold and Platinum, fibrinogen was reduced making heparin not required when used in cell culture media.
Moreover, hPL Gold Clinical is the first clinical grade hPL made from European donor material where no addition of heparin is required.
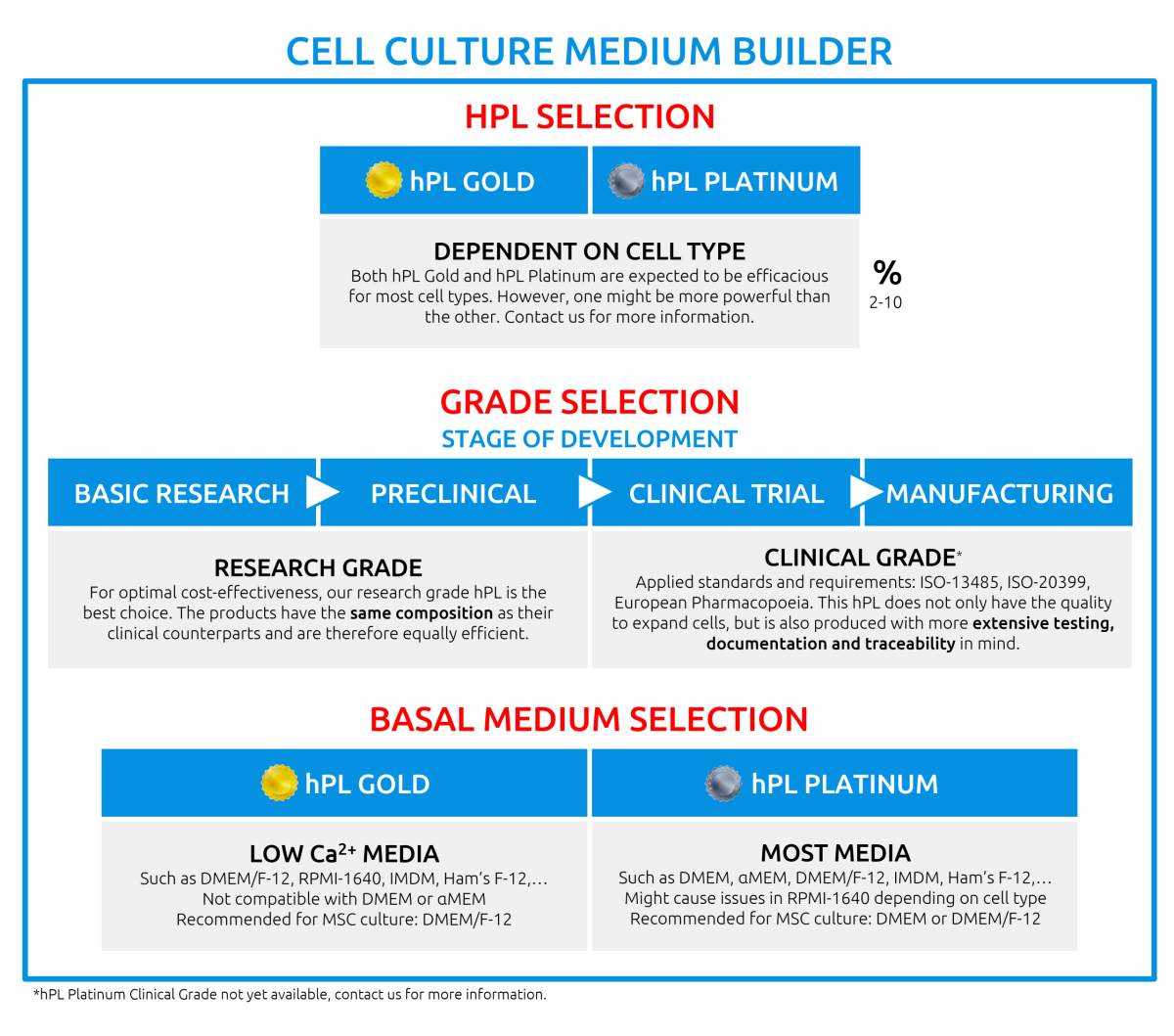
What types of hPL do you offer?
Currently, you can purchase hPL Gold for research purposes (G150R) or for clinical purposes (G150C). hPL Platinum is currently available for research purposes (P480R).
What is the difference between hPL Gold and hPL Platinum?
hPL Platinum is a next generation platelet lysate that contains an even higher concentration of growth factors, so the composition is different. For most cells, both hPL Gold and hPL Platinum can be used. For optimal selection of hPL, we recommend validating both products with your cells.
At what percentage should hPL be used?
Typically, hPL can be added to basal medium at 5 to 10%. In some situations, this can be reduced even further. However, for the optimal choice, it is always recommended to set up a validation comparing different concentrations. Do not hesitate to contact us, we will be happy to assist you.
What package sizes are available?
hPL Gold (G150R and G150C) is offered in 100mL bags, hPL Platinum (P480R) is offered in 180mL bags.
Are these products tested for pathogens?
Multiple layers of security were built in to offer the highest possible quality. Healthy donors are selected by a doctor using a medical questionnaire and all donations are screened for human immunodeficiency virus 1 and 2, Hepatitis C virus, Hepatitis B surface antigen and Treponema pallidum. Pathogen inactivation is performed on all platelet concentrates. More info on source product safety can be found at this link (Dutch).
Platelet concentrates are processed into hPL in a closed system avoiding ambient contamination. The final hPL undergoes additional tests (mycoplasma, endotoxin, bacteriological culture) and quality controls. The clinical grade versions were produced using following standards and requirements: ISO-13485, ISO-20399 and European Pharmacopoeia.
What basal medium should I use?
The choice of basal medium depends on your application, duration of cell culture and the medium supplements used. For hPL Gold, the use of DMEM/F-12, RPMI-1640, Ham’s F-12 and IMDM is advised. Combination with regular DMEM, MEM or similar are likely to cause precipitation and issues in your cell culture. If the use of DMEM or MEM is required, you can make use of a version without CaCl2. If not possible, hPL Platinum might be a better fit.
hPL Platinum has been validated for use with DMEM, MEM, αMEM, DMEM/F-12, Ham’s F-12 and IMDM. Other basal media should be tested for your purpose. Please do not hesitate to contact us, we are happy to assist you.
What is the shelf life of hPL?
hPL Gold can be stored for 2 years at -80°C. We recommend aliquoting the hPL upon receipt in volumes suitable for you to avoid unnecessary freeze-thaw steps.
I notice precipitation in my cell culture, what can I do?
If you see particles in your cell culture, this can mean several things. First of all, we recommend that you always filter your complete medium (i.e. after addition of hPL to the basal medium). If despite filtration you still see particles then you might be dealing with calcium complex precipitation, in this case we recommend using a different basal medium or working at a lower hPL concentration. Before taking these steps it is of important to check that your cell culture has not been contaminated.
It is not uncommon for the hPL to appear turbid or contain flocculation after thawing. Because the source product was prepared with transfusion purposes in mind and the preparation is done in a closed system, there is no need for sterile filtration as with many other commercial products. This eliminates the risk of losing important proteins that can be used in your cell culture. Moreover, all products underwent pathogen inactivation and of course it is also thoroughly checked before it reaches you. If you wish to remove flocculation from the undiluted hPL, this can be done by centrifugation.
Should I add heparin to my medium?
No, fibrinogen depletion is performed during the preparation of hPL. Thus, heparin is not required.
Are hPL Gold and hPL Platinum xeno-free?
Yes, hPL does not contain any animal products and they are not used during preparation. So our hPL is xeno-free.
History hPL
The story of Human Platelet Lysate (hPL) began in the 1960s, when fetal calf serum (FBS) was the dominant source of nutrition in culture media. However, research in the 1980s showed that platelet lysates could also serve as an alternative. With the development of cultured cells in vitro, safety concerns about FBS arose, leading to the rediscovery of platelet lysate as an alternative in the early 2000s. This shift was spurred by guidelines that sought to minimize the use of FBS in cell therapy. At the same time, blood banks worldwide have a surplus of platelet concentrates that cannot be used for transfusion.